Michaela Liedler, PT MSc1; Dipl-Ing. Dr.techn. Gebhard Woisetschläger
Citation / Download article as a PDF:
Liedler, M., & Woisetschläger, G. (2019). Influence of postoperative adhesions after caesarean section on chronic lower back pain – A pilot study of osteopathic manipulative treatment. European Journal of Osteopathic Research, 1(1), 38–46. https://doi.org/10.35740/EJOR.2019.1.1.5 Cite Download
Abstract
Background: Despite the prevalence of abdominal adhesions after a caesarean section, there exist few postoperative treatment approaches which specifically target adhesions or establish their connection with chronic lower back pain (cLBP).
Aims: To investigate if the osteopathic approach of treating adhesions after a caesarean section reduces existing cLBP symptoms and alleviates associated pain.
Methods: The subjects received two 30-minute treatments with a one week pause between treatments. The intervention group A (n=18), those who received osteopathic treatment, were compared to a control group B (n=16), who received scar treatment using traditional physiotherapy. The evaluation of subjective (pain intensity with a numeric rating scale, or NRS) and objective (symptom evaluation using the Oswestry Low Back Pain Questionnaire) parameters was accomplished using questionnaires before and after the treatments.
Results: Pain intensity reduced clinically relevant in group A by MA21=-2.6; SDA21=1.33 on the NRS. The average Oswestry Disability Index (ODI) in group A decreased from M1=18.3%; SD1=7.8 to M2=6.2%; SD2=6.2. In group B, the decrease from M1=19.1%; SD1=11.1 to M2=14.0%; SD2=10.1 was significantly smaller (p=0,005).
Conclusions: Postoperative adhesions could cause cLBP. Treatment of adhesions using osteopathy results in a significant reduction of pain symptoms for cLBP. Due to the sample size calculation, further studies addressing adhesions and chronic lower back pain would be recommended.
Keywords: peritoneal adhesions; visceral adhesions; caesarean section; chronic lower back pain (cLBP)
DOI: https://doi.org/10.35740/EJOR.2019.1.1.5
Background
Numerous studies show that peritoneal adhesions form after an abdominal operation in between 50% and 95% of cases [1–3]. Despite the development of various surgical and pharmacological methods to lower the risk of adhesions forming, long-term postoperative ailments, such as digestive issues, back pain and infertility, can be observed [2, 4, 5]. In the case of adhesions forming during the healing process of the peritoneum, growths form between the peritoneal fascia, which can harden into fibrous structures. This can result in loss of mobility and flexibility in nearby structures [6–8].
Manual therapy treatments for such adhesions are rarely found in existing research [9]. Chapelle and Bove dealt concretely with the visceral mobilisation of adhesions in studies with rats [9, 10], while in one pilot study, Probst examined the influence of osteopathic treatment on postoperative recovery after a gastrointestinal operation [11]. Several case studies and studies show that treatment of postoperative scars can reduce individual pain [12–14].
This study is concerned with the osteopathic treatment of postoperative adhesions after a caesarean section and the effect of such treatment on chronic lower back pain (cLBP) as compared to scar treatments using traditional physiotherapy.
Material and Methods
This single-blind, block randomised, clinical study examined two groups, namely, the intervention group A (osteopathy) and a control group B (physiotherapy), which received two 30-minute treatments one week apart. Group A was comprised of 18 subjects and group B of 16 subjects.
Evaluation of the subjective (pain intensity with a numeric rating scale, or NRS [15]) and objective (symptom evaluation using the Oswestry Low Back Pain Questionnaire [16, 17]) parameters via questionnaire was performed directly prior to the first treatment and one week after the second and final treatment.
Subjects were recruited via osteopathy colleagues and doctors, as well as the social media platform, Facebook. Of the 63 applicants, 34 subjects were selected based on inclusion and exclusion criteria.
The study included female subjects between the ages of 20 and 69 who had experienced chronic lower back pain symptoms lasting at least six months [18] and who had undergone a caesarean section as recently as one year ago and, at most, one other abdominal operation, such as a second caesarean section or an appendectomy. After signing a letter of consent, subjects were then block randomised using a lottery in which group A (osteopathic treatment) or B (physiotherapy) was assigned.
To prevent distortion of the results, exclusion criteria included more than two abdominal operations, presence of cancer, other physical or physiotherapeutic treatments during the study, and the use of analgesics and/or muscle relaxers as part of a chronic pain therapy during the study.
The designation of inclusion and exclusion criteria, as well as treatment in both groups, was performed by the author of this study.
All subjects in the study were blind to their corresponding treatment group, as was the statistician with regard to the data, which was neutral in nature (namely, group A and B).
The treatment received by group A encompassed three standard osteopathic techniques combined into the authors own scar treatment concept. These techniques are used to relieve adhesions in the tissue and to restore the gliding behaviour of individual layers of tissue with one another. The direct technique [19], with and without the aid of the hip [19], mobilised the adhesions, after which the myofascial release technique [19] was used in combination with fascial unwinding [19].
Group B was treated with a scar massage according to Thomson, as is taught at the Academy of Physiotherapy in Vienna. Thomson describes four techniques for use on the scarred skin and the upper layers of tissue [20].
Statistical methods
Statistical analysis for this study was completed by Dr. Gebhard Woisetschläger. For a description of the random sample, the results of the questionnaire prior to treatment were used, whereby Wilcoxon-Mann-Whitney (WMW) tests, were also used to determine the initial similarity of the two groups. The two-tailed WMW-tests were completed at a level of significance of α=0.05.
Changes after treatment were described using the differential values of the chosen parameters between the two periods of evaluation, which were subjected to WMW-tests (dependent variable: differential values; independent variable: group A or group B).
Individual categories of the Oswestry Low Back Pain Questionnaire were examined by descriptive statistics, in which the average impairment was more than 25%.
Due to both the means and standard deviations of the differential values in both groups, effect sizes were calculated according to Cohen and the sample size calculation was determined using the ARE method for α=0.05 and P=0.80.
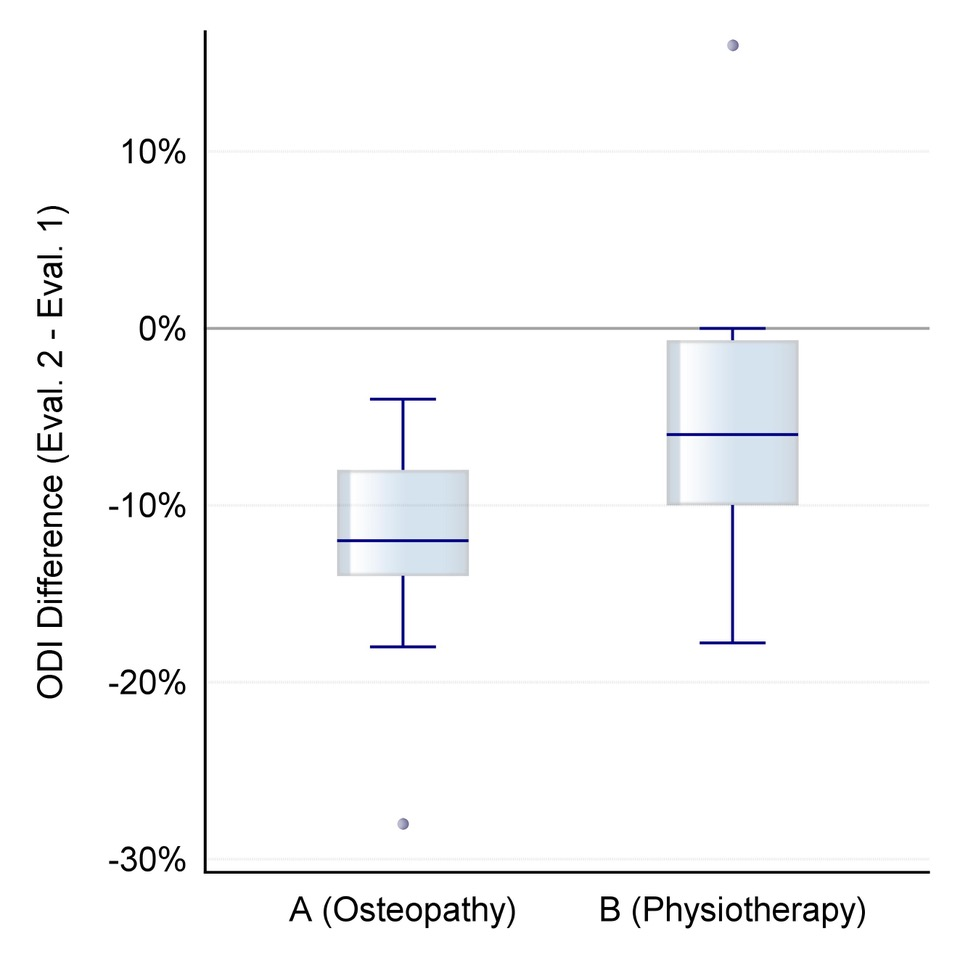 Figure 1. Distribution of differential values for change in self-assessed back pain intensity after the intervention on an NRS scale of 0-10 (orange: group A/osteopathy, green: group B/physiotherapeutic scar treatment).
Figure 1. Distribution of differential values for change in self-assessed back pain intensity after the intervention on an NRS scale of 0-10 (orange: group A/osteopathy, green: group B/physiotherapeutic scar treatment).
Results
In group A, the mean pain intensity reduced clinically relevant from MA1=4.6; SDA1=1.5 to MA2=2.0; SDA2=1.4 according to the NRS (0-10), and in group B from MB1= 5.1; SDB1=1.7 to MB2=3.7; SDB2=1.9.
The differential values between the second evaluation, after the intervention, and the first evaluation, prior to the intervention, were MA21=-2.6; SDA21=1.3 for group A and B MB21=-1.5; SDB21=1.9 for group B (W=80, p=0.068). The reduction in pain intensity was greater for group A, although the difference between groups was not significant.
Figure 1 shows the distribution of these differential values.
The mean Oswestry Disability Index in group A decreases from M1=18.3%; SD1=7.8 to M2=6.2%; SD2=6.2. In group B, the decrease from M1=19.1%; SD1=11.1 to M2=14.0%; SD2=10.1 is significantly smaller.
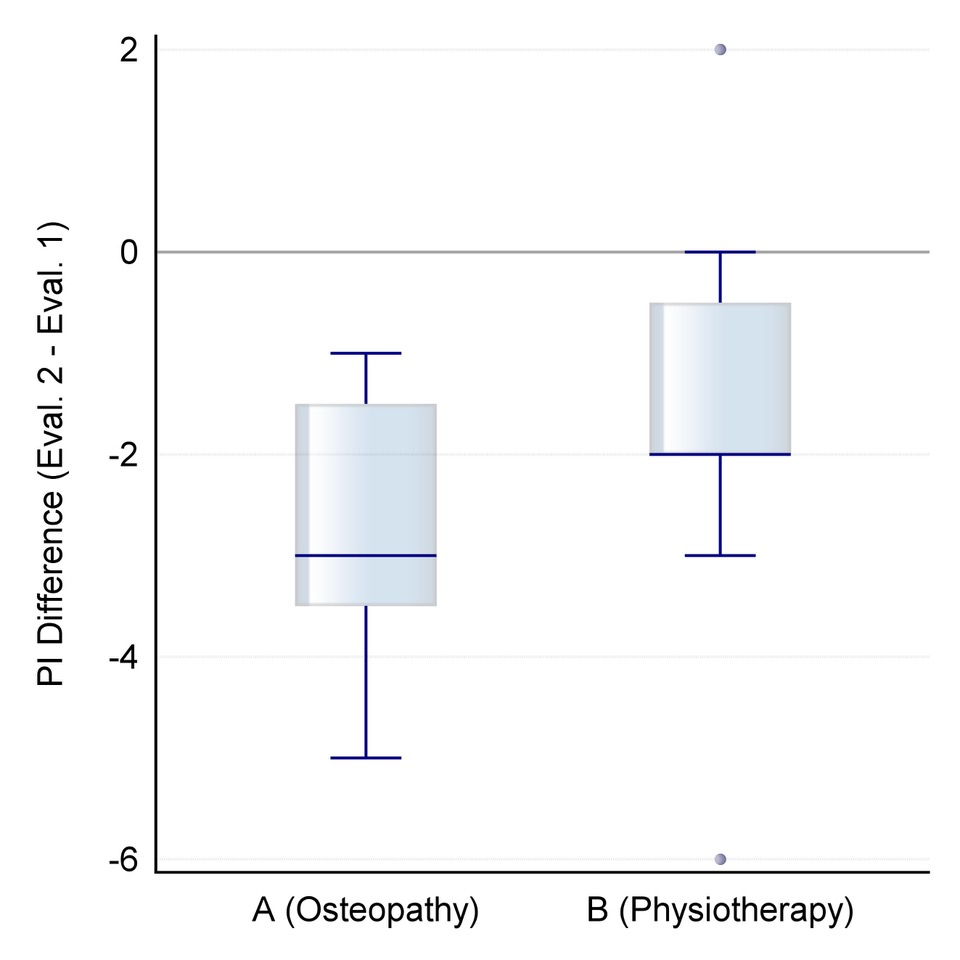 Figure 2. Distribution of differential values for change in the Oswestry Disability Index after the intervention (orange: group A/osteopathy, green: group B/physiotherapeutic scar treatment).
Figure 2. Distribution of differential values for change in the Oswestry Disability Index after the intervention (orange: group A/osteopathy, green: group B/physiotherapeutic scar treatment).
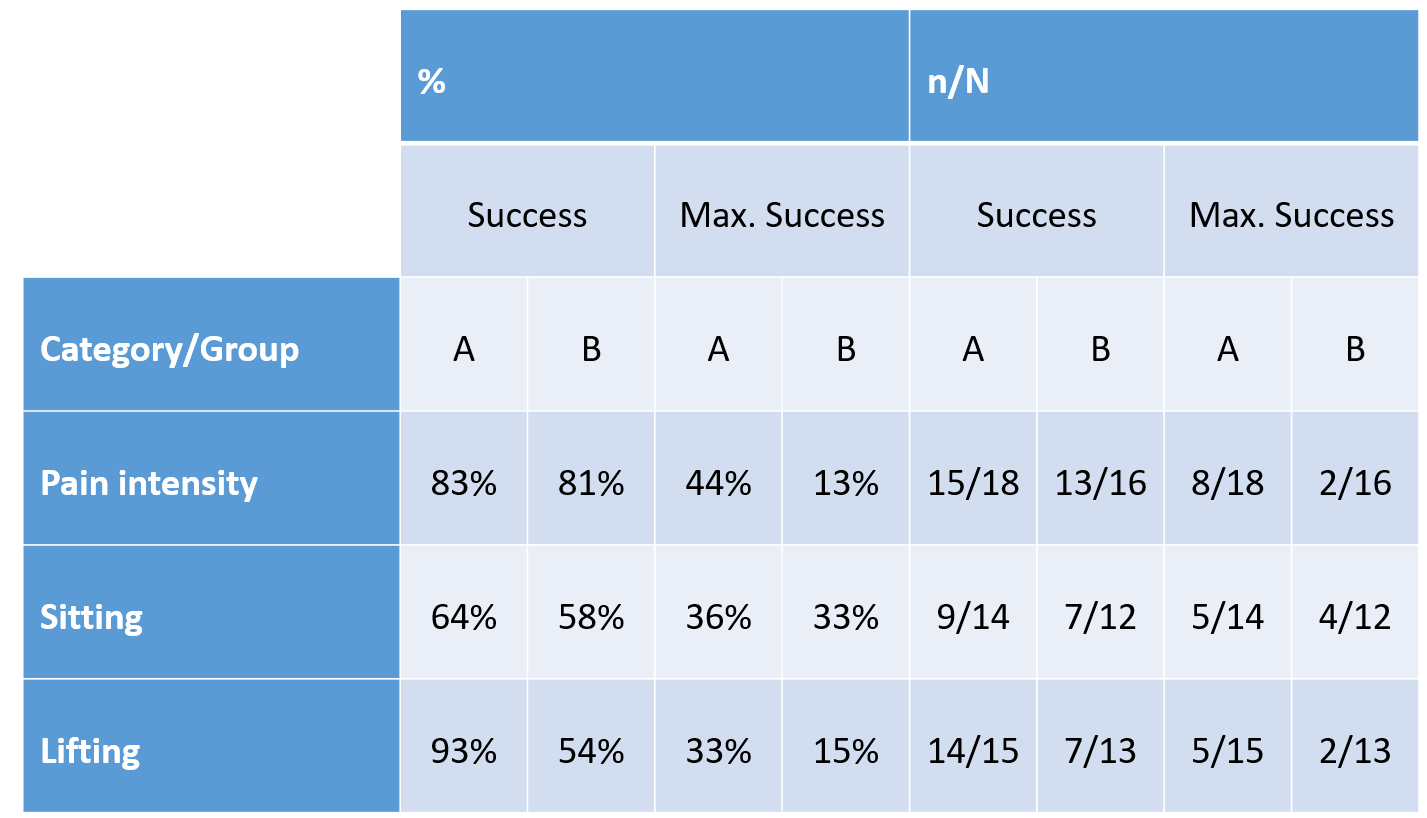 Table 1. Treatment success and maximum treatment success in the ODI categories „pain intensity“, „sitting“ and „lifting“ (n of N of impaired subjects prior to treatment)
Table 1. Treatment success and maximum treatment success in the ODI categories „pain intensity“, „sitting“ and „lifting“ (n of N of impaired subjects prior to treatment)
The mean differential value for group A M=-12.1%; SD=5.5 shows statistically significant lower negative values (W=62.5, p=0.0050) than those of group B, namely M=-5.1%; SD= 7.7, (see Figure 2).
The statistical power of p=0.66 was calculated post-hoc.
Most impairments were in the ODI categories „pain intensity“, „sitting“ and „lifting“. The success of the treatments in each group (reduction of impairment) and maximum treatment success (no impairment as of the second evaluation) can be seen in Table 1.
Discussion
In general, the osteopathic treatment of adhesions in subjects with chronic lower back pain after a caesarean section is more effective than the physiotherapeutic scar treatment according to Thomson, as is taught at the Academy for Physiotherapy in Vienna [20], as clearly shown by the total index of the ODI. A clinically relevant reduction in subjective pain intensity could be measured in both groups; by three points in group A and two points in group B on the NRS.
Group A, in which the focus lay on the adhesions, experienced a significant improvement in cLBP symptoms. Studies from Chapelle and Bove confirm that using visceral mobilisation, adhesions can be loosened [9] and the associated impact can be lessened [10]. Changes in the microstructure may lead to a modification of the macrostructure and allow the tissue to return to its physiological function [12]. The results of this study confirm this finding since both groups experienced a clinically relevant improvement in pain and Group A a significant decrease in symptoms.
In this study, direct, intermittent and pendulum techniques, as well as longitudinal and latitudinal gliding were used with the intention of improving the gliding layers [12], breaking up adhesions in the tissue, creating micro-fissures, and setting a localized inflammation process in motion [21–25] in order to increase collagenase production [26].
In addition, a mixture of MFR and FU was used to reduce pain and tightness in the tissues, to calm the tissue and inflammatory reaction [21] as the result of intense, mechanical stimulation [23, 24, 27–29], and to quicken the healing and rebuilding processes thereafter [30, 31].
At the same time, the pendulum-like motion impulses stimulate and ensure the release of peritoneal fluids and hyaluronan, which helps reduce friction between the organs and the peritoneal layers and facilitate gliding [32–34]. Gravitational forces, as they were used here, transfer forces not just longitudinally, but also to neighbouring tissues and adjacent structures [35, 36] and, via improved gliding surfaces in the visceral space, have a positive impact on their environment [29, 37].
Because the deep, transverse fascia, which is opened in the case of a caesarean section [38], is located directly next to the peritoneum [39], it is possible that the effects of adhesions could hinder its mobility [6] and that the transfer of force to neighbouring fascia and muscles would change [40] and stiffen [41]. Because of the anatomic proximity to the abdominal and back musculature [39], there may be a connection to the thoracolumbar fascia with its many mechanoreceptors [35, 39] and high pain sensitivity [42], which plays an important role in cLBP [37, 43, 44].
The limitations of this study are that all subjects were treated by the author and the lack of prior knowledge about the necessary sample size. Further studies using the sample size calculated in this study and various, neutral therapists for each treatment group are recommended.
Conclusions
Adhesions as postoperative complications are common in the medical field [1–3], and their widely varying effects on the rest of the body are often mentioned in the research [2, 4–8]. Various studies describe positive effects of postoperative scars on the body [11–14], but studies on the treatment of adhesions are hard to find [9, 10]. The results of the pilot study suggest that postoperative adhesions could cause cLBP, since the treatment of adhesions showed a decrease in cLBP. This is shown by the statistically significant ODI and by a clinically relevant reduction in cLBP pain intensity of group A. Due to these results, one can assume that the osteopathic treatment of adhesions and the restoration of gliding layers in the abdomen after a caesarean section would reduce pain and pain symptoms for cLBP.
Further studies using the calculated sample size and neutral therapists would be recommended to better establish this connection.
Discloser
The authors have no personal financial or institutional interest in any of the materials or devices described in this article.
Author details
1 Praxis Liedler, Austria
Correspondence
Michaela Liedler, Seuttergasse 17/1/7
1130 Wien, Austria
www.michaelaliedler.at
office@michaelaliedler.at
Published
29 December 2019, Accepted: 28 December 2019, Received: 01 December 2019
References
- Brüggmann, D., Tchartchian, G., Wallwiener, M., Münstedt, K., Tinneberg, H.-R., & Hackethal, A. (2010). Intra-abdominal Adhesions. Deutsches Aerzteblatt Online. https://doi.org/10.3238/arztebl.2010.0769 pp. 769–775
- Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery. (2007). Fertility and Sterility, 88(1), 21–26. https://doi.org/10.1016/j.fertnstert.2007.04.066
- Weibel, M.-A., & Majno, G. (1973). Peritoneal adhesions and their relation to abdominal surgery. The American Journal of Surgery, 126(3), 345–353. https://doi.org/10.1016/s0002-9610(73)80123-0
- Liakakos, T., Thomakos, N., Fine, P. M., Dervenis, C., & Young, R. L. (2001). Peritoneal Adhesions: Etiology, Pathophysiology, and Clinical Significance. Digestive Surgery, 18(4), 260–273. https://doi.org/10.1159/000050149
- Ten Broek, R. P. G., Issa, Y., van Santbrink, E. J. P., Bouvy, N. D., Kruitwagen, R. F. P. M., Jeekel, J., … van Goor, H. (2013). Burden of adhesions in abdominal and pelvic surgery: systematic review and met-analysis. BMJ, 347(oct03 1), f5588–f5588. https://doi.org/10.1136/bmj.f5588
- Arung, W. (2011). Pathophysiology and prevention of postoperative peritoneal adhesions. World Journal of Gastroenterology, 17(41), 4545. https://doi.org/10.3748/wjg.v17.i41.4545
- diZerega, G. S. (2001). Peritoneal repair and post-surgical adhesion formation. Human Reproduction Update, 7(6), 547–555. https://doi.org/10.1093/humupd/7.6.547
- D. Stanziu & D. Menzies, „The magnitude of adhesion-related problems“, Colorectal Disorders, vol. 9, nr. 2, pp. 35–38, 2007.
- Bove, G. M., & Chapelle, S. L. (2012). Visceral mobilization can lyse and prevent peritoneal adhesions in a rat model. Journal of Bodywork and Movement Therapies, 16(1), 76–82. https://doi.org/10.1016/j.jbmt.2011.02.004
- G. M. Bove, Susan L. Chapelle, K. E. Hanion, M. P. Diamond, D.J. Mokler, „Attenuation of postoperative adhesions using a mod-eled manual therapy“. PLoS One 12 (6). https://doi.org/e0178407, 2017.
- Probst, P., Büchler, E., Doerr-Harim, C., Knebel, P., Thiel, B., Ulrich, A., & Diener, M. K. (2016). Randomised controlled pilot trial on feasibility, safety and effectiveness of osteopathic MANipulative treatment following major abdominal surgery (OMANT pilot trial). International Journal of Osteopathic Medicine, 20, 31–40. https://doi.org/10.1016/j.ijosm.2016.03.002
- Chamorro Comesaña, A., Suárez Vicente, M. del P., Docampo Ferreira, T., Pérez-La Fuente Varela, M. del M., Porto Quintáns, M. M., & Pilat, A. (2017). Effect of myofascial induction therapy on post-c-section scars, more than one and a half years old. Pilot study. Journal of Bodywork and Movement Therapies, 21(1), 197–204. https://doi.org/10.1016/j.jbmt.2016.07.003
- Martínez Rodríguez, R., & Galán del Río, F. (2013). Mechanistic basis of manual therapy in myofascial injuries. Sonoelastographic evolution control. Journal of Bodywork and Movement Therapies, 17(2), 221–234. https://doi.org/10.1016/j.jbmt.2012.08.006
- Wasserman, J. B., Abraham, K., Massery, M., Chu, J., Farrow, A., & Marcoux, B. C. (2018). Soft Tissue Mobilization Techniques Are Effective in Treating Chronic Pain Following Cesarean Section. Journal of Womenʼs Health Physical Therapy, 1. https://doi.org/10.1097/jwh.0000000000000103
- Hawker, G. A., Mian, S., Kendzerska, T., & French, M. (2011). Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF. Arthritis Care & Research, 63(S11), S240–S252. https://doi.org/10.1002/acr.20543
- Mannion, A. F., Junge, A., Fairbank, J. C. T., Dvorak, J., & Grob, D. (2005). Development of a German version of the Oswestry Disability Index. Part 1: cross-cultural adaptation, reliability, and validity. European Spine Journal, 15(1), 55–65. https://doi.org/10.1007/s00586-004-0815-0
- Fairbank, J. C. T., & Pynsent, P. B. (2000). The Oswestry Disability Index. Spine, 25(22), 2940–2953. https://doi.org/10.1097/00007632-200011150-00017
- R. A. Deyo et al.., „Report of the NIH Task Force on Research Standards for Chronic Low Back Pain“, Physical Therapy, vol. 95, nr. 2, pp. e1–e18, 2015.
- W. Dough et al.., „Glossary of Osteopathic Terminology“, American Association Colleges Osteopaths Medicine, pp. 1-15, 2011.
- C. Preinfalk, „Scarmassage by Thomson“, Vienna, 2015.
- T. A. Wynn, „Cellular and molecular mechanisms of fibrosis“, Journal of Pathology, vol. 214, nr. 2, pp. 199–210, 2008.
- A. Kobesova & K. Lewit, „A case of a pathogenic active scar“, ACO, vol. 9, nr. 1, pp. 17–19, 2000.
- J. G. Dodd, M. M. Good, T. L. Nguyen, A. I. Grigg, L. M. Batia, & P. R. Standley, „In Vitro Biophysical Strain Model for Understanding Mechanisms of Osteopathic Manipulative Treatment“, Journal of the American Osteopathic Association, vol. 106, nr. 3, pp. 157–166, 2006.
- Standley, P. R., & Meltzer, K. (2008). In vitro modeling of repetitive motion strain and manual medicine treatments: Potential roles for pro- and anti-inflammatory cytokines. Journal of Bodywork and Movement Therapies, 12(3), 201–203. https://doi.org/10.1016/j.jbmt.2008.05.006
- A. J. Threlkeld, „The Effects of Manual Therapy on Connective Tissue“, Journal of the American Physical Therapy Association, nr. 72, pp. 893–902, 1992.
- A. Carano & G. Siciliani, „Effects of continuous and intermittent forces on human fibroblasts in vitro.“, European Journal of Orthodology, vol. 18, nr. 1, pp. 19–26, 1996.
- Hinz, B., Mastrangelo, D., Iselin, C. E., Chaponnier, C., & Gabbiani, G. (2001). Mechanical Tension Controls Granulation Tissue Contractile Activity and Myofibroblast Differentiation. The American Journal of Pathology, 159(3), 1009–1020. https://doi.org/10.1016/s0002-9440(10)61776-2
- Fernández-de-las-Peñas, C., Alonso-Blanco, C., Fernández-Carnero, J., & Carlos Miangolarra-Page, J. (2006). The immediate effect of ischemic compression technique and transverse friction massage on tenderness of active and latent myofascial trigger points: a pilot study. Journal of Bodywork and Movement Therapies, 10(1), 3–9. https://doi.org/10.1016/j.jbmt.2005.05.003
- Tozzi, P., Bongiorno, D., & Vitturini, C. (2011). Fascial release effects on patients with non-specific cervical or lumbar pain. Journal of Bodywork and Movement Therapies, 15(4), 405–416. https://doi.org/10.1016/j.jbmt.2010.11.003
- Cao, T., Hicks, M., Zein-Hammoud, M., & Standley, P. (2015). Duration and Magnitude of Myofascial Release in 3-Dimensional Bioengineered Tendons: Effects on Wound Healing. The Journal of the American Osteopathic Association. https://doi.org/10.7556/jaoa.2015.018
- Cao, T., Hicks, M., & Standley, P. (2013). In Vitro Biomechanical Strain Regulation of Fibroblast Wound Healing. The Journal of the American Osteopathic Association, 113(11), 806–818. https://doi.org/10.7556/jaoa.2013.056
- J. O. A. M. van Baal et al.., „The histophysiology and pathophysiology of the peritoneum“, Tissue Cell, vol. 49, pp. 95–105, 2016.
- R. Muts, „Treatment of peritoneal layers and organs.“, taught in osteopathic treatment concepts 12: Peritoneum, Vienna, 2015.
- G. S. DiZerega & K. E. Rogers, The Peritoneum, vol. 1. New York: Springer-Verlag, 1992.
- Schuenke, M. D., Vleeming, A., Van Hoof, T., & Willard, F. H. (2012). A description of the lumbar interfascial triangle and its relation with the lateral raphe: anatomical constituents of load transfer through the lateral margin of the thoracolumbar fascia. Journal of Anatomy, 221(6), 568–576. https://doi.org/10.1111/j.1469-7580.2012.01517.x
- Guimberteau, J. C., Delage, J. P., McGrouther, D. A., & Wong, J. K. F. (2010). The microvacuolar system: how connective tissue sliding works. Journal of Hand Surgery (European Volume), 35(8), 614–622. https://doi.org/10.1177/1753193410374412
- Tozzi, P., Bongiorno, D., & Vitturini, C. (2012). Low back pain and kidney mobility: local osteopathic fascial manipulation decreases pain perception and improves renal mobility. Journal of Bodywork and Movement Therapies, 16(3), 381–391. https://doi.org/10.1016/j.jbmt.2012.02.001
- Joura, E. A., & Husslein, P. (2000). A critical assessment of the Misgav-Ladach section technique. Der Gynäkologe, 33(4), 298–302. https://doi.org/10.1007/s001290050549
- W. Platzer, Atlas of Anatomy – Bewegungsapparat, 7. Aufl., vol. 1. New York: Thieme Verlag, 1999.
- Passerieux, E., Rossignol, R., Letellier, T., & Delage, J. (2007). Physical continuity of the perimysium from myofibers to tendons: Involvement in lateral force transmission in skeletal muscle. Journal of Structural Biology, 159(1), 19–28. https://doi.org/10.1016/j.jsb.2007.01.022
- Schleip, R., Naylor, I. L., Ursu, D., Melzer, W., Zorn, A., Wilke, H.-J., … Klingler, W. (2006). Passive muscle stiffness may be influenced by active contractility of intramuscular connective tissue. Medical Hypotheses, 66(1), 66–71. https://doi.org/10.1016/j.mehy.2005.08.025
- Schilder, A., Hoheisel, U., Magerl, W., Benrath, J., Klein, T., & Treede, R.-D. (2014). Sensory findings after stimulation of the thoracolumbar fascia with hypertonic saline suggest its contribution to low back pain. Pain, 155(2), 222–231. https://doi.org/10.1016/j.pain.2013.09.025
- Langevin, H. M., Stevens-Tuttle, D., Fox, J. R., Badger, G. J., Bouffard, N. A., Krag, M. H., … Henry, S. M. (2009). Ultrasound evidence of altered lumbar connective tissue structure in human subjects with chronic low back pain. BMC Musculoskeletal Disorders, 10(1). https://doi.org/10.1186/1471-2474-10-151
- H. M. Langevin et al.., „Reduced thoracolumbar fascia shear strain in human chronic low back pain“, BMC Musculoskeltal Disorders, vol. 12, nr. 203, pp. 1–11, 2011.
Rights and permissions
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license https://creativecommons.org/licenses/by/4.0/, and indicate if changes were made.
